产品简介
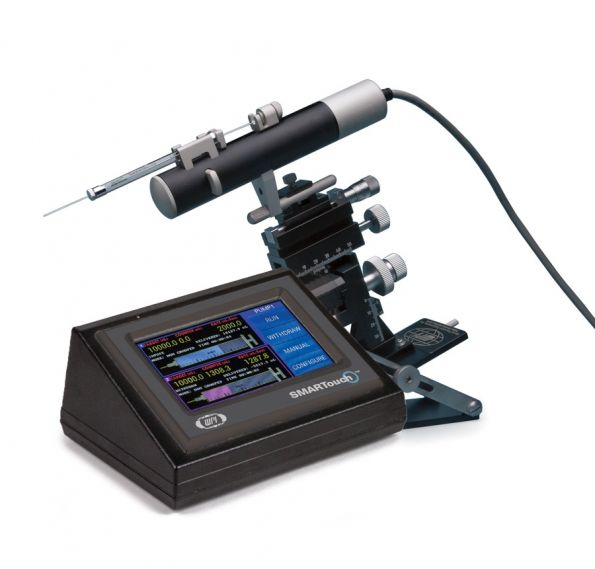
UMP3是显微操作系统市场中的第一款超微量操作泵,设计改良确保UMP3成为立体定位仪脑内注射应用中的最佳选择。配合触摸屏微电脑控制器SMARTouch™使用,能够达到更加直观、智能的控制效果。
触摸屏微电脑控制器SMARTouch™提供了一个智能又方便操作的界面,可以同时或者单独控制两个UMP3。控制屏幕以图形的方式显示了UMP3超微量显微操作泵的电源注射量和剩余量,以及其它相关的注射参数,比如注射泵的分组状态、目标注射量、注射速度、已注入的量、注射泵的运行时间等。
通过触摸屏,您可以手动或自动地运行注射泵,改变泵的方向,或者访问其它界面。通过轻触控制屏幕上一个按钮,就可以访问所有配置参数,您可以设置这些参数。控制器后部的USB端口可用来与计算机连接,从而进行远程控制。

产品特征
1、全新Micro2T触屏控制器与UMP3的配套具有完美特征:
2、智能化的触屏控制器具有图形显示操作界面,简单操作,方便使用;
3、单个触屏控制器可以同时控制两个UMP3注射泵; 触屏控制器屏幕具有防溅水能力;
4、触屏控制器具有双模式马达驱动功能; 具有手动和自动注射两个可选项;
5、具有防止注射泵过度运行的终点设置功能,有效延长泵体的使用寿命;
6、用于神经电生理研究注射时,具有超安静的功能, 防止对生理电信号的干扰;
其它产品特征包括:
*注射精度高:使用体积较小的微量注射器可以精确到皮升级别;
*通过支点的改进, 使得UMP3无论是固定在小动物脑立体定位仪,还是显微操作器上都可以稳定安全运行;
*兼容WPI公司和其它品牌的国际标准注射器, 大小包括0.5uL,1uL,5uL,10uL,25uL,50uL,100uL, 250uL,500uL和1000uL的微量注射器;
*可以在手动显微操作器如M3301,M325,KITE, MMJ等上运行;也可以在502300和502600等脑立体定位仪上运行; 还可以配合SN-PCZ-50R压电显微操作器上运行;


产品用途
1、小动物研究:
与脑立体定位仪配合使用,可以用于光遗传研究中病毒和荧光染料的颅内注射;
与脑立体定位仪配合使用,可以用于动物行为学研究中神经递质或药物的颅内注射;
2、斑马鱼研究:
与微量注射器配合使用可以用于斑马鱼成鱼体内药物或荧光染料的注射;
与IO-KIT或RPE-KIT等结合使用,可以转换成玻璃毛细管注射针头,从而用于斑马鱼幼鱼的体内药物或荧光物质的注射;
3、眼科研究:
与IO-KIT或RPE-KIT等结合使用,可以用于小动物眼内或视网膜色素上皮的药物注射或染料注射;
4、微流控研究:
通过选用合适的注射器或适合的注射速度,可以用于微流控研究。
5、化学研究:
可以有效控制注射速度,用于化学工程中药物或底物的添加。


UMP3超微量显微操作泵参考文献
[1] In vivo functional diversity of midbrain dopamine neurons within identified axonal projections eLife. 2019; 8: e48408.
https://doi.org/10.7554/eLife.48408
[2] Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum eLife. 2019; 8: e46764.
https://doi.org/10.7554/eLife.46764
[3] The Glutamatergic Postrhinal Cortex–Ventrolateral Orbitofrontal Cortex Pathway Regulates Spatial Memory Retrieval Neuroscience Bulletin 2019, 35(3):447–460
[4] Artificially Enhancing and Suppressing Hippocampus-Mediated Memories Current Biology 2019, 29(11):1885-1894.e4
https://doi.org/10.1016/j.cub.2019.04.065
[5] An ER Assembly Line of AMPA-Receptors Controls Excitatory Neurotransmission and Its Plasticity Neuron 2019, 104(4): 680-692.e9
https://doi.org/10.1016/j.neuron.2019.08.033
[6] Engram Cell Excitability State Determines the Efficacy of Memory Retrieval Neuron 2019, 101, 1–11
https://doi.org/10.1016/j.neuron.2018.11.029
[7] A Rare Mutation of β1-Adrenergic Receptor Affects Sleep/Wake Behaviors Neuron 2019, 103(6):1044-1055.e7
https://doi.org/10.1016/j.neuron.2019.07.026
[8] Engram Cell Excitability State Determines the Efficacy of Memory Retrieval Neuron 2019, 101(2):274-284.e5
https://doi.org/10.1016/j.neuron.2018.11.029
[9] Hypothalamic neuronal circuits regulating hunger-induced taste modification Nature Communications 2019, 10, 4560
https://doi.org/10.1038/s41467-019-12478-x
[10] Aversive state processing in the posterior insular cortex Nature Neuroscience 2019, 22:1424–1437
https://doi.org/10.1038/s41593-019-0469-1
[11] Interplay between α2-chimaerin and Rac1 activity determines dynamic maintenance of long-term memory Nature Communications 2019, 10, 5313
https://doi.org/10.1038/s41467-019-13236-9
[12] Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli Nature 2018, 397–401
https://doi.org/10.1038/s41586-018-0682-1
[13] In vivo base editing of post-mitotic sensory cells Nature Communications 2018, 9, 2184 https://doi.org/10.1038/s41467-018-04580-3
[14] Spectrally distinct channelrhodopsins for two-colour optogenetic peripheral nerve stimulation Nature Biomedical Engineering 2018, 2:485–496
https://doi.org/10.1038/s41551-018-0255-5
[15] Sexual rejection via a vomeronasal receptor-triggered limbic circuit Nature Communications 2018, 9, 4463
https://doi.org/10.1038/s41467-018-07003-5
[16] Microglia permit climbing fiber elimination by promoting GABAergic inhibition in the developing cerebellum Nat Commun. 2018; 9: 2830.
https://doi.org/10.1038/s41467-018-05100-z
[17] Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions Nature Neuroscience 2018, 21:1431–1441
https://doi.org/10.1038/s41593-018-0228-8
[18] Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning Cell 2018,173(6):1329-1342.e18
https://doi.org/10.1016/j.cell.2018.04.004
[19] Neural ensemble dynamics underlying a long-term associative memory Nature 2017, 543:670–675
https://doi.org/10.1038/nature21682
[20] Early hyperactivity and precocious maturation of corticostriatal circuits in Shank3B−/− mice Nature Neuroscience 2016, 19:716–724
https://doi.org/10.1038/nn.4260
[21] Cortico-fugal output from visual cortex promotes plasticity of innate motor behaviour Nature 2016, 538:383–387
https://doi.org/10.1038/nature19818
[22] Locus coeruleus and dopaminergic consolidation of everyday memory Nature 2016, 537:357–362
https://doi.org/10.1038/nature19325
[23] The calcium sensor synaptotagmin 7 is required for synaptic facilitation Nature 2016, 529:88–91
https://doi.org/10.1038/nature16507
[24] Activating positive memory engrams suppresses depression-like behaviour Nature 2015, 522:335–339
[25] A circuit mechanism for differentiating positive and negative associations Nature 2015, 520:675–678
[26] Projections from neocortex mediate top-down control of memory retrieval Nature 2015, 526:653–659
https://doi.org/10.1038/nature15389
[27] Decoding Neural Circuits that Control Compulsive Sucrose Seeking Cell 2015, 160(3):528-541
https://doi.org/10.1016/j.cell.2015.01.003
[28] Natural Neural Projection Dynamics Underlying Social Behavior Cell 2014, 157(7):1535-1551
https://doi.org/10.1016/j.cell.2014.05.017